Inclusion Conjunctivitis And Trachoma are chronic infectious diseases of the eye and genital tract,caused by closely related microorganisms and exhibiting overlapping spectra of clinical manifestations and epidemiologic patterns. These range from mild and self-limited to severe and blinding eye disease, with or without involvement of the genital tract.
Etiology of Inclusion Conjunctivitis And Trachoma
The agents of trachoma and inclusion conjunctivitis (TRIC agents) are members of the psittacosis-lymphogranuloma venereum-trachoma group. Formerly these agents were considered viruses; now they are called chlamydiae. They are nonmotile, gram-negative, obligate intracellular parasites which multiply in the cytoplasm of their host cells by a distinctive developmental cycle.
Chlamydiae differ from viruses in many important respects: they possess both RNA and DNA; they multiply by binary fission; they possess bacterial types of cell walls and ribosomes; they produce a variety of metabolically active enzymes; and their growth can be inhibited by several antibacterial drugs. It is probable that chlamydiae are closely related to gram-negative bacteria, but lack some significant mechanism for the production of metabolic energy so that they are restricted to an intracellular existence.
TRIC agents can infect epithelial cells of conjunctiva, cornea, urethra, and cervix of man and monkey, but do not replicate in most other tissues of the body. The infective particle (“elementary body”) is a sphere with a diameter of 250 nanometers (millimicrons) and stains purple with Giemsa’s or red with Macchiavello stain. The infective particle is taken into the host cell by phagocytosis and, after replication, results in the development of an inclusion body. This is -an oval or crescent shaped mass of “elementary bodies” embedded in a matrix of glycogen, lying in the cytoplasm of an epithelial cell often adjacent to the nucleus. Inclusion bodies can be stained brightly with specific immunofluorescence.
TRIC agents can be grown in the yolk sac of embryonated eggs and in X-irradiated cells in culture. Only a few laboratory strains replicate freely in cell cultures. The growth of TRIC agents is markedly inhibited by tetracyclines, sulfonamides,erythromycin, penicillins, and chloramphenicol, but not by aminoglycosides. Therefore streptomycin or neomycin can be used to suppress bacterial contaminants in clinical specimens and to aid in isolation of TRIC agents in the laboratory.
TRIC agents share with other chlamydiae a heat-stable group antigen. In addition, species- or strain-specific antigens have been detected in the cell wall. Infected persons may develop antibodies to both types of antigens. TRIC agents, like other chlamydiae, contain a toxin which kills mice within a few hours after intravenous injection. Repeated injection of chlamydial suspensions can protect mice against the homologous toxin. Such toxin-protection tests indicate the presence of several antigenic types among TRIC agents, and provide the main available method for antigenic classification of new isolates.
Thus, genital TRIC isolates often fall within certain antigenic groups, whereas. ocular TRIC isolates from endemic trachoma areas often fall within other groups. Antigenic classification of strains is also being attempted with fluorescent antibody methods. However, to date there is no specific laboratory characteristic that can always differentiate isolates of trachoma from isolates of inclusion conjunctivitis agents.
TRIC agents can persist in man for years, with or without symptoms and signs of infection. Sub- clinical infection can be activated by trauma, corticosteroid administration, and perhaps bacterial superinfection. The development of scarring and blindness in some endemic trachoma regions is attributable to a large extent to recurrent bacterial infections of the eye, especially with Haemophilus or Neisseria.
Prevalence and Epidemiology.
Since antiquity, trachomatous infection was recognized in the Mediterranean basin and in the Orient. Today, trachomatous eye disease is very prevalent in Africa and Asia. It has been estimated that up to 400 million persons may be infected with TRIC agents, and up to 20 million may be economically blinded as a result. Trachoma flourishes in areas that are hot and dry, and that have a shortage of available water and poor hygienic customs. In such areas endemic levels are high, and initial infection commonly occurs in early childhood.
In certain parts of the world, e.g., in North Africa, virtually the entire population is infected with TRIC agents before reaching adulthood. The high prevalence of bacterial superinfection in such populations undoubtedly contributes to the severity of eye disease and to its causing widespread blindness. In parts of the United States, e.g., Indian reservations of the Southwest, endemic TRIC infection is relatively common, but it rarely leads to major visual impairment, perhaps because bacterial superinfection is infrequent. Throughout the world sporadic cases of ocular
TRIC infection occur, often with a clinical picture resembling trachoma. It is probable that some of these originate in genital infections rather than in contact with ocular trachoma.Active cases of ocular TRIC infection shed the infective agent in desquamated conjunctival cells, conjunctival exudate, or tears. The infectious agent may be transmitted by fingers, fomites, and perhaps flies. Patients with early active cases probably shed more infective TRIC agent, and thus are more infectious for contacts than are those with chronic cases. However, even patients with long-standing scarred eye disease, and without signs of current activity, may shed TRIC agent and thus serve as a source of infection.
These comments concern ocular TRIC infection, particularly typical endemic trachoma. Typical inclusion conjunctivitis has a radically different epidemiologic pattern.- Inclusion conjunctivitis is fundamentally an infection of the adult human genital tract, transmitted as a venereal disease. In the female the TRIC agent grows mainly in the transitional epithelium of the cervix. These cells may contain typical inclusions, and occasionally a mild cervicitis is present. In the male, genital infection may produce urethritis, as the TRIC agent replicates in urethral epithelium. In both sexes, adult genital tract infection is often asymptomatic.
Adults may transfer genital secretions to their own or other eyes by fingers, by fomites, and—occasionally —in swimming pools. The infection may pass from the cervix of the infected mother to the eye of her newborn during passage through the birth canal. The newborn infant may then develop eye disease between 6 and 14 days after birth.
The epidemiologic patterns described for typical trachoma and typical inclusion conjunctivitis may be mixed. Thus, the genital tract of women in endemic trachoma regions may harbor TRIC agents, and these may produce either inclusion conjunctivitis or clinically typical trachoma in the offspring. Genital isolates from adults in non endemic areas may, upon inoculation into the eye, give rise to disease resembling either inclusion conjunctivitis or trachoma.
Pathology and Pathogenesis of Inclusion Conjunctivitis And Trachoma.
TRIC agents invade mainly the epithelium of the conjunctiva, cervix, and urethra. The earliest sign of infection is the appearance of inclusion bodies within epithelial cells and neutrophil infiltration of the epithelium of mucous membranes. Subepithelial infiltration with plasma cells and lymphocytes and the development of lymphoid follicles then follow. In the cornea, epithelial keratitis is often accompanied by the formation of subepithelial opacities. Blood vessels from.the limbus, accompanied by fibroblasts, may invade the cornea to form a pannus. Progression of the inflammatory process leads to necrosis and scar formation in the conjunctiva. In classic trachoma, these changes occur more markedly in the upper half of the conjunctival sac and cornea; in inclusion conjunctivitis, more markedly in the lower conjunctiva.
Inflammatory changes, lymphoid infiltration, necrosis, and scar formation have also been observed in TRIC agent infection of cervix and urethra.Classic inclusion conjunctivitis of the newborn is an acute purulent conjunctivitis; in the adult it is a follicular conjunctivitis. However, all pathologic changes from limited follicular conjunctivitis to full-blown “trachoma” with scars and pannus may follow inoculation of genital isolates into the adult eye.Classic, chronic, severe trachoma in hyperendemic areas progresses to marked lid deformation and loss of vision.
As a result of cellular infiltration and scarring near the lid margins, the tarsal plate is bowed and the lid margins inverted (entropion), and some eyelashes turn inward (trichiasis), rubbing against the cornea with each lid movement, and aggravating corneal opacification. The scarring may destroy tear function, with resulting keratinization of corneal epithelium.TRIC agents exhibit a pronounced tendency to persist in infected tissues for years even in the absence of signs of active disease, and this predisposes to relapses and to chronicity.
Clinical Manifestations of Inclusion Conjunctivitis And Trachoma.
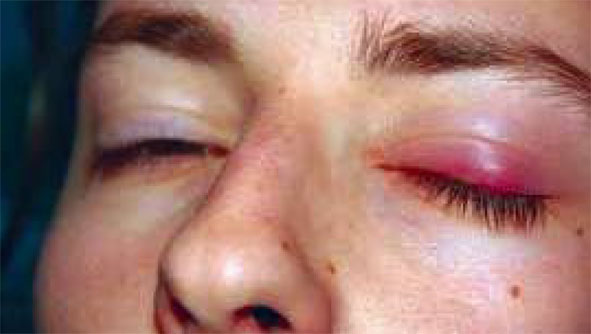
In experimental human infection with TRIC agents the incubation period is from two to seven days. Inclusion conjunctivitis of the newborn usually begins between the fifth and fourteenth days of . life (whereas gonococcal ophthalmia usually begins two days after birth). The incubation period of naturally occurring trachoma is uncertain because the onset is often insidious, particularly in children. Early symptoms of TRIC infection are those of irritation, lacrimation, and mucopurulent discharge. The earliest physical signs are conjunctival hyperemia, mucopurulent discharge, papillary hypertrophy in infants, and follicular hypertrophy in adults.
Typical trachoma in children and adults begins as a follicular conjunctivitis, most noticeable in the conjunctiva of the upper lid and the tarsal plate. There are epithelial keratitis and subepithelial corneal infiltration, followed by gradual corneal vascularization from the upper limbos downward. This evolves into a dense fibrovascular pannus extending over part, or all, of the cornea, grossly impairing vision.
Linear or stellate scars appear on the conjunctiva. Progressive scarring of the subepithelial tissues leads to deformation of the tarsal plate, resulting in entropion. trichiasis, and further corneal damage These changes often follow secondary bacterial: which may also produce corneal accelerate loss of vision. Typically systemic symptoms or signs of ini eye is the sole involved organ-
Typical inclusion conjunctivitis presents as an acute purulent papillary hypertrophy but litEfeet the cornea. Over a period of weefc tends to regress scenarios-1;. heal without anv scars or widu :The use of chemical skin cleaners may leave a residue that contains virus-inactivating material, and vigorous physical cleansing of the site may create minute abrasions that then can become sites of secondary vaccinia eruptions, with resultant involvement of a comparatively large skin area.
Vaccination Technique.
Regardless of age, routine primary vaccination should be performed with no more than two or three pressures with the side of a needle. These pressure points should be as close together as possible, and should be made only at one site. With the highly potent vaccines currently in use, more numerous pressure points are not necessary and certainly should not be utilized for a nonimmune person. When children or adults are to be revaccinated after a lapse of more than five years, the same small number of pressure points should be used. For re vaccination within a five-year period of those persons known to have had a major reaction, the full complement of 30 strokes can safely be used (Leake, 1946).
Vaccination Reaction.
The description of the reactions after vaccination or revaCcination should follow the criteria recommended by the Expert Committee on Smallpox of the World Health Organization. A successful primary vaccination is one that on examination after seven to ten days presents a typical jennerian vesicle. If this is not present, vaccination must be repeated with fresh vaccine and a few more strokes of the needle. The successful revaccination is one that on examination one week (six to ten days) later shows a vesicular or pustular lesion or an area of definite palpable induration and congestion surrounding a central lesion; this lesion may be a scab or an ulcer. These reactions are termed major reactions; all others should be called equivocal reactions.
A major reaction indicates virus multiplication with consequent development of immunity. An equivocal reaction may merely represent an allergic response, which could be elicited by inactive vaccine or poor technique in someone who had been sensitized by earlier vaccination; or the equivocal reaction may result from sufficient immunity to prevent virus multiplication.
Since the allergic response cannot be readily differentiated from the one caused by true immunity another vaccination should be performed using a different lot of vaccine if there is the possibility that the first was of weak potency, and the procedure should be completed with an additional number of pressures. The site should be examined one week later; if the result is again equivocal, revaccination should be repeated using a full 30 pressures, as recommended by Leake. For the sake of expediency, an equivocal reaction to revaccination with a minimal insertion may be followed by vaccination at two sites, not less than two inches apart, using known potent vaccine. This method will make a third return unnecessary in almost all instances.
Frequency of Vaccination.
Re-vaccination is essential to reinforce the immunity conferred by previous vaccination. This not only maintains a high level of immunity against smallpox, but minimizes the risk of complications on revaccination. To maintain adequate immunity against smallpox, revaccination should be carried out at approximately five-year intervals. Those persons at some increased risk, such as hospital personnel and public health personnel, members of the armed forces, and those working at port or airline offices as well as shipping personnel should be revaccinated at least once every three years. When exposure to smallpox is probable, by travel or residence in a smallpox-endemic area, annual revaccination is desirable.