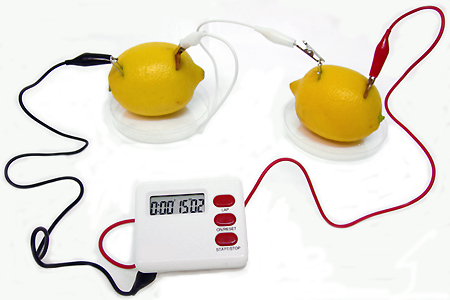
A lemon battery produces electricity through a chemical reaction that occurs when metals are introduced into the lemon juice. The lemon juice is quite acidic, which helps break the atomic structure of metals, such as copper and zinc, releasing electrons that generate electrical current.
The lemon battery is a science experiment that is used to demonstrate the basic concepts of a chemical battery system . To do this experiment, a scientist needs to start a lemon, and galvanized steel nail with a zinc cover and a copper coin. You also need a cable with clips at each end and a voltmeter. Other devices can also be used to test these batteries.
The lemon contains a good amount of juice that is acid, which gives the term electrolyte. The acid in an electrolyte helps break down the atomic structure of metals, such as copper and zinc, which causes individual electrons to be released. When a person creates a circuit by connecting the two metals with a conductor, the electrons flow through their electrical charge, which is detected by a voltmeter or other device.
Step By Step Guide To Make Lemon Battery
Step 1: Materials
The materials to use:
- Various Lemons
- Copper coin
- Zinc nail.
- Lizard Tweezers.
Step 2: Create The Battery
- Insert the nail or screw on the side of the lemon.
- . On the other side the coin, being always in contact the two metals by means of the juice of the lemon. This will be the battery.
- This will be done successively with the lemons you have.
- You can split or use the whole lemon.
Step 3: Connection
The connection will be as follows: From lemon 1 the coin is connected to the screw of lemon 2, then from lemon 2 the screw is connected to the coin of lemon 3 and then all the lemons are connected.
You have to make sure that:
- The copper wires are well attached to the nails. They should make good contact.
- The nails and the bare parts of the copper wires are quite submerged and do not touch.
Creating a lemon battery is a classic science experiment used to demonstrate how a simple chemical reaction can create electricity. Here’s a guide in tabular format that explains how it works:
| Step | Description |
|---|---|
| 1. Gather Materials | – Lemon – Copper coin or strip – Zinc nail or galvanized nail (coated in zinc) – Wires – LED or small bulb (optional) |
| 2. Insert Electrodes | Insert the copper and zinc electrodes into the lemon, ensuring they do not touch. The lemon juice acts as an electrolyte. |
| 3. Chemical Reaction | The acid in the lemon juice reacts with the zinc, causing electrons to move from the zinc to the copper. This is because zinc is more reactive and loses electrons more readily than copper. |
| 4. Electron Flow | The movement of electrons from the zinc to the copper through the wires creates an electric current. |
| 5. Close the Circuit | Connect the wires to an LED or a small bulb to close the circuit. The flow of electrons will light up the LED/bulb. |
| 6. Multiple Lemons for More Power | To increase voltage, you can connect multiple lemon batteries in series. Each lemon adds about 0.9 volts to the circuit. |
Remember, the power generated by a single lemon battery is quite small, so it’s usually not enough to power larger devices. The experiment mainly serves as an educational tool to illustrate basic principles of chemistry and electricity.